News
New product upgrade announcement November 10 2020
New product upgrade announcement
Plastic full-length disposable stethoscope cover: Now self-sealing with built-in adhesive strip and convenient dispenser box.
Product images and information are below.

Disposable Plastic Full-Length Covers 100ct - $24.00
Plastic full-length cover is recommended for droplet precautions/COVID-19.
Better than a disposable stethoscope: Cost-effective and enables providers to use their own high quality stethoscope outfitted with a cover.
This polyethylene sheath is 60cm long. Adhesive strip is built-in for each disposable sleeve. One size fits all stethoscopes. StethoBarrier does not impair sound transmission.
This cover is a single-use cover to reduce stethoscope-mediated pathogen transmission.
Compatible with the Eko DUO electronic stethoscope. Visit support.ekohealth.com for instructions on how to use StethoBarrier with this device.
This cover system is patented.
For large orders, vendor onboarding, or to place a purchase order please contact us at StethoBarrier.com.
SKU: 00861300000127
Made in USA
All StethoBarrier products are in stock:
Plastic full length disposable stethoscope cover
Online purchases may be made through our website. To set up an account for purchase orders contact us at info@stethobarrier.com or via our contact form.
We are here to help and support our front line providers as you care for patients during this time.
October 2020 Updates October 21 2020
October 2020 updates: All products in stockPersonal Protective Equipment and COVID-19 updates July 05 2020
While the supply of personal protective equipment (PPE) has improved over time, judicious use of PPE remains critical as cases of COVID-19 in many portions of the United States are starting to surge. For those trying to determine how many units of StethoBarrier sleeves or other equipment to order, the CDC has released a Burn Rate Calculator to assist in inventory management:
https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/burn-calculator.html
COVID-19, product shortages and personal protective equipment guidelines May 11 2020
Judicious use of personal protective equipment continues to be critical as the number of cases of COVID-19 continue to increase and hospitals are strategically ramping up routine and elective care.
We are continuing to supply and re-stock the plastic full length disposable stethoscope cover as this cover is splash proof and our recommended cover for droplet precautions/COVID-19.
Quantities of non-woven covers are limited at this time.
If you have a product availability inquiry submit your question at www.stethobarrier.com/pages/
We are here to help and support our front line providers as you care for patients during this time.
Links to resources on the COVID-19 outbreak and PPE management from credible sources are accessible below:
CDC Strategies to Optimize the Supply of PPE and Equipment
https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html
CDC Healthcare Supply of Personal Protective Equipment
https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html
CDC Healthcare personnel guidelines for caring for patients with COVID-19
https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-patients.html
WHO site on the Coronavirus disease (COVID-19) outbreak
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
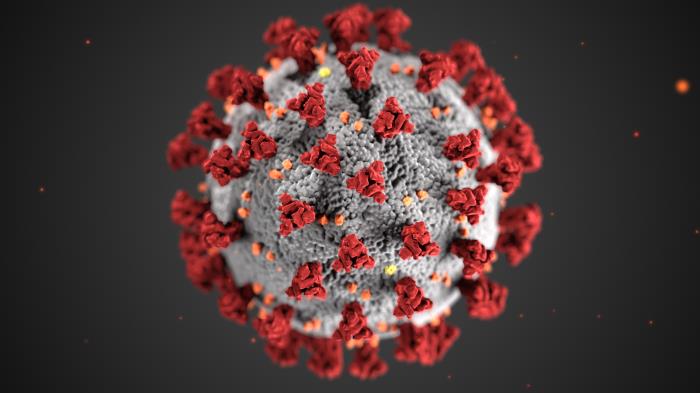
Photo credit:
CDC/ Alissa Eckert, MS; Dan Higgins, MAM. Centers for Disease Control and Prevention's Public Health Image Library (PHIL), identification number #23312.
This illustration, created at the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by coronaviruses. Note the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed electron microscopically. A novel coronavirus, named Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China in 2019. The illness caused by this virus has been named coronavirus disease 2019 (COVID-19).
Novel coronavirus (COVID-19) and personal protective equipment guidelines March 04 2020
With increasing numbers of cases of COVID-19 virus identified globally and in the United States, the World Health Organization (WHO) and Centers for Disease Control (CDC) are releasing guidelines on screening, personal protective equipment and caring for patients with the novel coronavirus (see below).
In addition to COVID-19, we are still in the midst of cold and flu season. We recommend the following general guidance for all healthcare employees and the general public:
- Wash hands frequently with soap and water for at least 20 seconds or use an alcohol-based hand rub with at least 60% alcohol. Always wash hands when visibly soiled.
- Wash hands prior to touching your eyes, nose, or mouth.
- When in contact with those who are sick, wear the appropriate personal protective equipment
The CDC and WHO are reporting increased demand and plans for surge manufacturing globally to prevent supply shortages.
StethoBarrier is not currently experiencing supply shortages for any of our disposable stethoscope covers. We will update our news section should this change.
Links to resources on the COVID-19 outbreak from credible sources are accessible below:
CDC Healthcare Supply of Personal Protective Equipment
https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html
CDC Healthcare personnel guidelines for caring for patients with COVID-19
https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-patients.html
WHO site on the Coronavirus disease (COVID-19) outbreak
https://www.who.int/emergencies/diseases/novel-coronavirus-2019

Photo credit:
CDC/ Alissa Eckert, MS; Dan Higgins, MAM. Centers for Disease Control and Prevention's Public Health Image Library (PHIL), identification number #23312.
This illustration, created at the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by coronaviruses. Note the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed electron microscopically. A novel coronavirus, named Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China in 2019. The illness caused by this virus has been named coronavirus disease 2019 (COVID-19).
Centers for Disease Control and Prevention Releases Updated Guidelines for Prevention of Surgical Site Infections May 08 2017
The Centers for Disease Control and Prevention recently released updated guidelines for prevention of surgical site infections. This is a highly anticipated update as the last set of guidelines was released in 1999. As noted in the invited commentary in JAMA, “Surgical Site Infection Prevention—What We Know and What We Do Not Know,” by Dr. Pamela A. Lipsett, “there is a lot of opportunity to learn how we can provide more effective care to our patients.”
While evidence-based medicine remains the gold standard, unfortunately, in many instances there is lack of quality evidence utilizable for evaluating the benefits, harms and tradeoffs of various interventions. While there are definitive recommendations in the guidelines, the authors conclude by stating that the proposed evidence-recommendations should be incorporated into comprehensive quality improvement programs in order to improve patient safety. StethoBarrier strongly commends the Centers for Disease Control and Prevention for their efforts to systematically review the literature and concisely summarize their recommendations.
The findings and conclusion from the report is excerpted below, please use the link in the full citation below to access the full report.
Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017
Findings Before surgery, patients should shower or bathe (full body) with soap (antimicrobial or nonantimicrobial) or an antiseptic agent on at least the night before the operative day. Antimicrobial prophylaxis should be administered only when indicated based on published clinical practice guidelines and timed such that a bactericidal concentration of the agents is established in the serum and tissues when the incision is made. In cesarean section procedures, antimicrobial prophylaxis should be administered before skin incision. Skin preparation in the operating room should be performed using an alcohol-based agent unless contraindicated. For clean and clean-contaminated procedures, additional prophylactic antimicrobial agent doses should not be administered after the surgical incision is closed in the operating room, even in the presence of a drain. Topical antimicrobial agents should not be applied to the surgical incision. During surgery, glycemic control should be implemented using blood glucose target levels less than 200 mg/dL, and normothermia should be maintained in all patients. Increased fraction of inspired oxygen should be administered during surgery and after extubation in the immediate postoperative period for patients with normal pulmonary function undergoing general anesthesia with endotracheal intubation. Transfusion of blood products should not be withheld from surgical patients as a means to prevent SSI.
Conclusions and Relevance This guideline is intended to provide new and updated evidence-based recommendations for the prevention of SSI and should be incorporated into comprehensive surgical quality improvement programs to improve patient safety.
Full citation:
Surgeons of the US Navy demonstrating maintenance of a sterile field.
Impact of healthcare provider awareness on hand hygiene June 18 2016
We have long known that healthcare associated infections lead to negative outcomes, increased costs and dissatisfaction with the healthcare system, yet there are many occasions when providers fail to wash their hands and sanitize small medical equipment. As healthcare providers, we never intend to do harm to our patients or actively think to not take precautions. Rather, we are focused on fulfilling other responsibilities and tasks pertaining to patient care and in a rush or moment of forgetfulness we neglect infection control basics. These lapses highlight the importance of a systems-based approach to ensure infection control practices are routinely implemented.
At StethoBarrier we view our products as a visual reminder of stethoscope hygiene and believe that offering a safe alternative to low quality disposable stethoscopes will lead to fewer protocol violations where providers use their own stethoscope on contact isolation patients.
Henry Ford Health System recently took an interesting approach of showing staff members the millions of bacteria that can grow off of common surfaces including unused gloves, doorknobs, a nurse station mouse, health-care workers' hands, a mobile phone and an ultrasound machine.
Their study was inspired by a previous study that was designed to provoke a feeling of disgust in healthcare workers as a means of motivating them to wash their hands. Following posting of the images, the study found that every participating unit experienced at least an 11% increase in hand-washing, with one unit improving by nearly 50%.
Read more on CNN, “This is what made doctors wash their hands” by Janissa Delzo.

Photo Credit: CNN, Bacteria found on at ultrasound machine
Infection Control: 2015 in Review December 29 2015
In 2015 infection control continued to be of paramount importance in hospital operations and entered the news headlines on multiple occasions. From concern about superbug infections associated with duodenoscopes to ebola and resurgence of vaccine preventable illness, infection control has entered mainstream health conversations across the nation. As an organization committed to helping healthcare facilities minimize healthcare associated infections, we challenge our partners and clients to continuously evaluate ways in which we can come together to promote the adoption of policies that improve patient safety - whether its being more careful about hand and equipment sanitation or taking the time to ensure patients understand the risks and benefits associated with declining vaccines. Below we have shared a link to the Becker's Infection Control & Clinical Quality review of the top stories of 2015 including increased plague infections, duodenoscope sanitation debate, misdiagnosis in the headlines, increased cases of Legionnaires disease and NTM infections as a risk associated with open-heart surgery.
Infection control in the US: 2015 year in review
In a year when quality and infection control in healthcare has been top-of-mind for healthcare executives as these two elements are increasingly tied to their organizations' bottom lines, it was difficult to choose just five events to highlight in this "year in review" piece. But the Becker's Clinical Quality and Infection Control editorial team did just that.
For reference, we did something similar in the middle of 2015. In that piece, we listed the following five stories:
- Superbug infections linked to duodenoscopes
- Hospital Compare star ratings launch
- Risk of low-volume surgeries uncovered
- Ebola treatment and preparation in the U.S.
- Antibiotic resistance in focus
Building on those, here are five more infection control and patient safety stories from 2015 that affected hospitals, resulted in changed processes or uncovered new safety risks. They are presented in no particular order.
Plague on the rise
Even though many people consider the plague to be a medieval problem, the U.S. has seen several plague cases within its borders this year. The plague is caused by the bacterium Yersinia pestis and generally spreads to humans when they are bit by a rodent flea or they handle an animal with the plague. According to the CDC, 16 cases of plague have been reported in the U.S. through Nov. 16, and four of those people have died.
That is well above the usual number of U.S. plague cases: According to CDC data, the annual number of plague cases has ranged from one to 17 between 2001 and 2012, with the median being just three cases per year.
"It is unclear why the number of cases in 2015 is higher than usual," the CDC said in a report issued at the end of August, but it did encourage healthcare workers to consider a plague diagnosis when patients present with common signs or symptoms of plague, have traveled to the western U.S., and have been in proximity with rodents and their habitats or with ill domestic animals.
Fortunately, since the dawn of the antibiotic era, plague mortality has fallen from as high as 93 percent down to 16 percent. The key to survival is early treatment with an antibiotic such as aminoglycosides, fluoroquinolones or doxycycline....Continue reading at Becker's Infection Control & Clinical Quality website.
Influenza, Stethoscopes and Infection Control September 28 2015
As the fall and winter months arrive we will begin to see influenza cases around the United States. Remember, your stethoscope is a critical fomite in infection control. See below for CDC recommendations on how to protect patients from Influenza during their physician’s visits:
“Fundamental Elements to Prevent Influenza Transmission
Preventing transmission of influenza virus and other infectious agents within healthcare settings requires a multi-faceted approach. Spread of influenza virus can occur among patients, HCP, and visitors; in addition, HCP may acquire influenza from persons in their household or community. The core prevention strategies include:
- administration of influenza vaccine
- implementation of respiratory hygiene and cough etiquette
- appropriate management of ill HCP
- adherence to infection control precautions for all patient-care activities and aerosol-generating procedures
- implementing environmental and engineering infection control measures.
Successful implementation of many, if not all, of these strategies is dependent on the presence of clear administrative policies and organizational leadership that promote and facilitate adherence to these recommendations among the various people within the healthcare setting, including patients, visitors, and HCP. These administrative measures are included within each recommendation where appropriate. Furthermore, this guidance should be implemented in the context of a comprehensive infection prevention program to prevent transmission of all infectious agents among patients and HCP.”
Prevention Strategies for Seasonal Influenza in Healthcare Settings: Guidelines and Recommendations. CDC. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm
Further resources available at:
Siegel J, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. 2007. http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/isolation2007pdf.
Why don't providers sanitize stethoscopes? August 02 2015
What prevents providers from sanitizing stethoscopes? A study of pediatric healthcare providers published in The American Journal of Infection Control found reasons for not sanitizing the stethoscope include concern about stethoscope wear and tear, lack of access to disinfection materials, lack of visual reminders and lack of time.1
StethoBarrier products address these concerns by providing small and full-length disposable stethoscope covers that do not damage the stethoscope, are easy to apply, and provide a visual reminder of stethoscope hygiene – our covers are bright blue so providers will notice if their stethoscope is missing one. Furthermore, disposable covers can improve quality of care while reducing supply costs by replacing low-quality disposable stethoscopes with providers’ high-quality stethoscopes outfitted with a cover.
- Predictors of stethoscope disinfection among pediatric health care providers. Muniz, Jeanette et al. American Journal of Infection Control, Volume 40, Issue 10, 922 - 925
Question: Why not use alcohol wipes? May 17 2014
Today we are writing about a commonly asked question - why can't doctors, nurses and caretakers just use alcohol wipes to clean off the stethoscopes as they go from patient to patient.
This is a great question and we are happy to answer. Overall, there are lots of germs and pathogens that alcohol is NOT effective against. This includes bacteria like Clostridium difficile which causes 14,000 American deaths each year. Individuals at the highest risk include patients taking antibiotics and receiving medical care - hence why this is a common Healthcare Associated Infection.
Alcohol damages stethoscopes. Dr. Didier Pittet, the leader in stethoscope transmission research, posses a question: How to clean the stethoscope without damaging it? “If I show you actually my own stethoscope, the stethoscope that I use for more than 30 years and that I used to actually cleanse, you can see that the stethoscope has been highly damaged so we need to find a way to cleanse the stethoscopes without damaging them.”
StethoBarrier protects patients from dangerous pathogens and protects physician’s stethoscopes from harsh disinfectants.
Viruses without an outer envelope are also unbothered by alcohol. For example, norovirus is the most common cause of acute gastroenteritis in the US. Each year, it causes 19-21 million illnesses and contributes to 56,000-71,000 hospitalizations and 570-800 deaths according to the Centers for Disease Control. Norovirus can be spread through contaminated food or water, or by touching contaminated surfaces. A study found that using alcohol sanitizers was linked to an INCREASE in norovirus cases in hospitals in the New England region: Click here to read the research study.
In conclusion, alcohol does not work against many organisms. Furthermore, alcohol is known to dry rubber and can damage the seals around the stethoscope's diaphragm, as well as the diaphragm itself. It is recommended that alcohol be used on a surface for at least 15 seconds in order to be effective, while StethoBarrer can be applied in under 5 seconds. Other studies have shown that one of the reasons healthcare workers say they forget to clean their stethoscope is because there is no visual reminder: Click here to read the research study.
StethoBarrier solves that problem - we are blue and either the doctor or the patient will notice if it is missing!